The passage of the Drug Price Competition and Patent Term Restoration Act in 1984 and its subsequent amendments (collectively the Hatch-Waxman Act) gave rise to more competition in the pharmaceutical industry and a new era of litigation. The act itself provides a mechanism for generic drug companies to quickly gain approval to sell a generic version of an existing brand name drug.
The application that begins the FDA approval process for the generic firm is called an Abbreviated New Drug Application (ANDA). Brand name drug manufacturers have an understandable incentive to delay approval of the ANDA. Simply, if the ANDA approval is delayed, the brand name firm continues to enjoy the lawful ability to sell their brand name drug without a lower priced generic equivalent in the market. One lawful mechanism brand name manufacturers use that may have the effect of delaying the approval of an ANDA is the filing of a Citizen Petition with the FDA.
The Citizen Petition filed by a brand name firm would typically allege that the proposed generic drug is not equivalent and thus should not be approved for sale. Should the Citizen Petition be deemed only a mechanism for delaying approval of the ANDA/generic drug rather than one filed with the public's health interest at heart, the brand name firm would be liable for antitrust violations.
Such was the question our firm faced when working on behalf of a brand name pharmaceutical firm recently. A Citizen Petition had been filed and a jury was going to be asked whether it had been lawfully filed. Were the jury to find that the Citizen Petition had been unlawfully filed with the intent to simply delay approval of the generic drug, they could possibly award hundreds of millions of dollars in damages. One quirk in this case that proved advantageous was the fact that it was not the generic drug firm suing the brand name firm, but instead it was the middleman or drug wholesaler who was alleging antitrust violations.
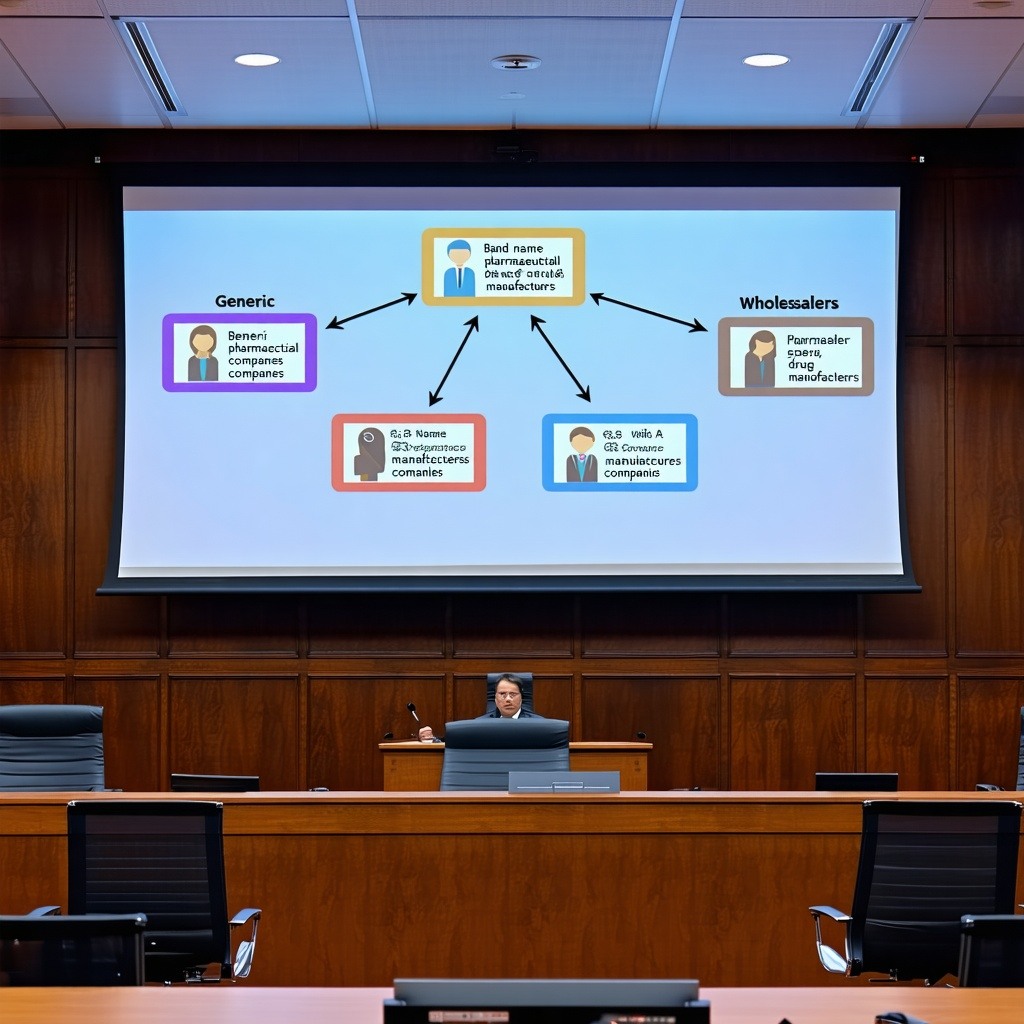
Our challenge in creating an effective trial presentation was to create trial exhibits that both taught the jury and persuaded the jury simultaneously. The trial exhibits shown below were part of an opening PowerPoint presentation that explained who was involved in the case (i.e. the typical parties/players trial exhibit) and who was not involved. We sought to emphasize that the brand name firm was being sued not by the generic drug manufacturer but instead the wholesaler who we painted as the delivery guys in these opening trial exhibits. The story told is this:
- Brand name firms seek approval for a new drug from the FDA;
- Brand name firms distribute their product through wholesalers who then sell them to pharmacies;
- Generic firms receive approval to sell through an ANDA;
- The brand name firm here is BrandName Pharma, generics will be mentioned and then there are the wholesalers. In this case HatchWax Wholesale Drug;
- One would think the generics are involved, but they are not. Only the wholesalers or the delivery guys are suing. What business do they have suing?
- Who is HatchWax Wholesale Drug? They are professional antitrust plaintiffs.
These trial exhibits shown in video format were part of a PowerPoint created for opening statements. Scroll below the movie for the impressive result.
Despite a serious threat with potential damages approaching half a billion dollars, our top five law firm client prevailed with the assistance of our trial exhibits. We received a complete defense verdict and our client noted about Animators at Law:
"The whole team was incredibly thoughtful, creative and always willing to answer the call. We would not hesitate to recommend you to any of our colleagues, we had a far better experience with you than with others we have used in the past (who didn't quite "get" what we were trying to convey and were always three steps behind us - you guys were consistently one step ahead)."





Leave a Comment